Lecanemab, which has been hailed as a breakthrough treatment for Alzheimer’s disease, has recently attracted significant attention from the media and healthcare professionals. However, existing data and recent analyses suggest a more nuanced picture than the initial hype might have suggested.
What is lecanemab?
Lecanemab is a drug developed to slow the progression of Alzheimer’s disease, a condition that affects memory and cognitive functions. It has been promoted as a "wonder-drug" due to its potential to slow cognitive decline in patients with early Alzheimer’s disease.
What does the data show?
Recent analyses, including a report from Japan’s PMDA, reveal several important points. The clinical trials for lecanemab tested various dosing regimens and assessed its effects at different time points.
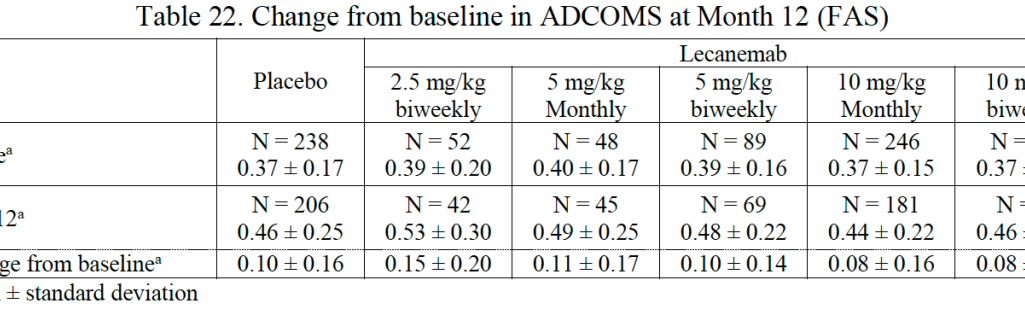
At 12 months, the most promising dosing regimen (10 mg/kg biweekly) demonstrated only a 64% chance of being superior to placebo by a clinically meaningful margin, falling short of the 80% success criterion set for the trial. At 18 months, changes in the Alzheimer’s Disease Composite Score (ADCOMS) were relatively modest across all treatment groups, with no dose achieving a significant advantage over the placebo.
In terms of safety, lecanemab has been linked to several adverse events, including infusion reactions and amyloid-related imaging abnormalities (ARIA), which can be serious and affect a considerable proportion of patients. During the open-label extension (OLE) phase of the study, 20.6% of participants experienced infusion reactions, even among those who initially received a placebo.
The trials evaluated multiple outcomes across different doses and time points, raising concerns about the potential for findings to be influenced by chance. The statistical analysis used to assess efficacy had to account for numerous comparisons, which can affect the reliability of the reported results.
Regulatory and financial implications
Despite the high-profile marketing of lecanemab, health authorities such as the National Institute for Health and Care Excellence (NICE) have deemed it not to offer good value for money, primarily due to its cost relative to the observed benefits. The high price of the drug could impose a significant financial burden on patients and healthcare systems, especially given the mixed results concerning its effectiveness. As with Comirnaty, Oxycontin, etc., pharmaceutical companies stand to make extraordinary fortunes by successfully selling their medicines as wonder-drugs, and can afford to have their products presented positively in the press.
Conclusion
While lecanemab represents a notable development in the treatment of Alzheimer’s disease, it is important to view such 'wonder-drugs' with a critical perspective. The available data indicates that while lecanemab may offer some benefits, these are limited and come with considerable safety concerns. Patients should consult with their healthcare providers to fully understand the implications of new treatments and make informed decisions based on the latest evidence.
Credit to Tom Jefferson and Carl Heneghan of Trust the Evidence for drawing our attention to this one.